Molecular Detection of Feline Herpesvirus by Means Polymerase Chain Reaction- Juniper Publishers
Journal of Dairy & Veterinary Sciences- Juniper Publishers
Abstract
In the Feline Respiratory Complex, the Feline
herpesvirus is a main etiological agent with worldwide distribution and
affect domestic cats causing ocular and upper respiratory tract lesions.
In Chile, only clinical diagnostic is used, unlike use several
techniques in other countries and Polymerase Chain Reaction (PCR) is the
most recommended with best performing of them. For implemented an
effective diagnostic method in our country, we select cats under
one-year-old with clinical signs suggest feline herpesvirus infection,
to detect thymidine kinase protein gen of herpesvirus, highly specific
and conserved, by a nested PCR and subsequent determination of
nucleotide identity percentage respect GenBank®, genomic database.
As a result, we obtained a high detection rate (100
percent of samples and positive control), and a 92 percent of nucleotide
identity in comparison with de genomic database GenBank® (accesion
number E12463.1), proving that correspond to feline herpesvirus. Thus,
achieved described an effective diagnostic method to be used as a
complementation of clinical diagnostic.
Keywords:Feline herpesvirus; Nested PCR; Thymidine kinase; Feline herpesvirus diagnosis
Abbreviations:
FHV: Feline Herpes Virus; Kb: kilobases; AV: Viral Isolation; IFI:
Indirect Immunofluorescence; SN: Seroneutralization; ELISA:
Enzyme-Linked Immune Sorbent Assay; PCR: Polymerase Chain Reaction
Introduction
Feline herpes virus type 1 (FHV-1) was isolated in
1957 by Crandell and Maurer [1]. It is a DNA virus linear double strand
containing approximately 134 kilobases (kb), subdivided into long and
short components of 104 and 30 kb respectively [2,3], which is immersed
in a capsid of icosahedral symmetry constituted by 162 capsomeres.
Surrounding the capsid is the tegument, an amorphous matrix that
contains globular proteins with enzymatic activity. Outside the tegument
is a pleomorphic lipoprotein envelope that presents spicules, many of
which are responsible for inducing an immune response in the host [4].
FHV-1 is widely distributed in the world, with approximately 90% of cats
being seropositive to the virus [5,6]. Only one serotype is known,
which differs in virulence depending on the strain [7]. In adult cats it
causes high morbidity and low mortality, whereas in small cats it can
generate a mortality of 60% [8].
FHV-1 belongs to the order Herpesvirales,
Herpesviridae family, Alphaherpesvirinae subfamily and Varicellovirus
genus [9]. The viruses of this subfamily are characterized by being
highly species-specific, have a short replicative cycle of 24 hours,
labile to the environment and common disinfectants, possess a marked
tropism to the epithelia and generate latency in neural tissue [10,11].
The domestic cat is the main host of FHV-1. The route of entry of the
virus is nasal, oral or conjunctival, causing multifocal
epithelial necrosis, with neutrophilic infiltration and inflammation in
conjunctiva, pharynx, trachea, bronchi to bronchioles [7]. The lesions
are caused by two mechanisms: direct, product of viral replication that
leads to cytolysis, and indirect, through the action of inflammatory
cells [6,11]. It does not cause viremia, except in neonates or in
hypothermic puppies, since viral replication occurs preferably at low
temperatures. Viral excretion begins 24 hours after the infection
occurs, and is maintained for one to three weeks, while the acute
clinical picture resolves within 10 to 14 days in immunocompetent cats
[12].
This virus causes latency in the trigeminal ganglion
mainly and 80% of infected cats become carriers for life [13,14]. The
reactivation of the virus can be induced experimentally by treatment
with glucocorticoids. However, there are other factors that can
reactivate the virus through stress such as: transfer to a new
environment (18% of cases), childbirth and breastfeeding (in 40% of
cases) [7]. In this way, small cats acquire the virus very early
(Conjuntitivis neonatorum) [8]. The development of the disease depends
on the level of maternal antibodies that they possess, which provide
passive immunity via colostral during the first weeks of life. When high
levels of antibodies are present, kittens are protected from the
disease and develop a subclinical infection that may end up becoming
latent. When there are not enough maternal antibodies, the clinical
disease develops. Unfortunately,
herpes virus infection does not provide a strong active immune
response, so infected females do not always have adequate
antibodies. Therefore, the immune response protects against the
disease, but not against the infection [7]. The prevalence of the
virus can range between 1-20% depending on the size of the cat
population. In catteries, the risks are greater, product of the high
animal density and the bad hygienic conditions [7,15].
Clinical Signs
The clinical illness can be divided into: Classic acute illness:
very severe in cats under one year of age. They present fever,
rhinitis, conjunctivitis, corneal ulcers both superficial and deep,
abundant mucopurulent secretion, both nasal and ocular and
recurrent sneezing. This leads to depression and anorexia. Atypical
acute illness: they present oral and nasal ulcers, dermatitis with
crusted lesions or pneumonia. Chronic illness: they present
stromal keratitis with corneal edema, vascularization, corneal
ulcers can evolve to corneal sequestration, blindness or a chronic
rhinosinusitis with nasal discharge and sneezing for life.
Some adult cats may develop lesions due to viral reactivation,
which is called recrudescence. They may present clinical signs
associated with both an acute condition and may progress to a
chronic condition [7,15]. There is often coinfection with feline
calicivirus and/or Chlamydophila felis, Bordetella bronchiseptica,
Mycoplasma sp, Staphylococcus sp, or Escherichia coli, causing
a multi-causal feline respiratory syndrome. There are vaccines
against FHV-1, but it has been shown that they do not provide
complete protection in all cats, especially in places with high
animal density, where viral load is high [10,16].
Diagnosis
The primary infection with FHV-1 is so aggressive that the
clinical diagnosis is simple. In contrast, in adults the clinical
signs in its chronic phase are mild and diverse, which makes viral
identification necessary [17]. Among the differential diagnoses of
an individual with rhinosinusitis, are: viral (Herpes, Calicivirus),
bacterial (Chlamydophila felis, Bordetella bronchiseptica,
Mycoplasma sp. as primary agent), fungal (Cryptococcus
neoformans, Aspergillus spp, Histoplasma capsulatum, Blastomyces
dermatitidis), parasitic (Capillaria aerophila, Syngamus ierey),
foreign bodies, allergies, dental alterations, polyps, neoplasms
(adenocarcinoma, for example), lymphoplasmacytic rhinosinusitis
and trauma [18]. Similar is the case of a cat with conjunctivitis,
whose differential diagnoses are: viral (Herpes, Calicivirus,
feline immunodeficiency virus, feline viral leukemia), bacterial
(Chlamydophila felis, Bartonella sp., Mycoplasma sp.), Food allergy,
atopy, traumatic (eyelash changes, irritant factors, etc.) [17].
There are several diagnostic methods such as: viral isolation
(AV), indirect immunofluorescence (IFI), seroneutralization (SN),
enzyme-linked immune sorbent assay (ELISA) and Polymerase
Chain Reaction (PCR). Compared with these, PCR is 25% more
sensitive than viral isolation from conjunctival scrapings,
presumably due to an inactivation of viral infectivity because the
envelope is easily destroyed by transport, freezing, enzymes or
antibodies present in saliva or tea [8,19]. In addition, it does not
need the virus to be viable and its results are not altered using vital
dyes such as fluorescein (necessary to diagnose corneal ulcers).
Unfortunately, it is not known if individuals vaccinated against
HVF-1 produce false positives when sampled and subjected to
PCR. A study carried out in 2011 concludes that after vaccinating
healthy individuals, both nasally and parenterally, a low
percentage of positives is obtained when using the PCR method.
However, only two vaccines were studied, so future research
is needed to clarify this question [20]. As for other diagnostic
techniques, such as AV and IFI, they have a high sensitivity in
cases where the clinical picture is acute, but not in chronic or
recrudescent cases [8]. Another disadvantage that has IFI is to be
a very subjective technique to depend on the experience of the
operator, and without good results, due to the low amount of viral
antigen and interference by the immune response, either humoral
or mediated by inflammatory cells, which increases the possibility
of false negatives [21]. In addition, IFI, SN and AV are procedures
that require a longer time and are expensive compared to PCR.
For these reasons, PCR is the method of choice for the
detection of FHV-1. Variations of conventional PCR have been
used: nested or in real time, the latter being the most modern and
with the capacity to provide additional information, for example:
a high number of copies suggests active replication, whereas a
low number indicates an infection latent with FHV-1. The samples
used to perform the PCR are diverse and have in common the
integration of different tissues where FHV-1 latency. The main
detection sites are: trigeminal ganglion, optic nerve, olfactory
bulbs, cornea and nasal turbines. Less frequently, the virus is
detected in: salivary and lacrimal glands, oral cavity, tonsils and
conjunctiva. The latter are more used since their extraction is not
necessarily post-mortar, but by scraping, brushing or swabbing
tissue. The most widely used technique worldwide now amplifies
a segment of the gene that codes for the protein Thymidine Kinase
[2]. It has the characteristic of being highly divergent in its amino
acid sequence between the different species of herpes viruses,
and extremely conserved among the isolates of FHV-1 [2]. Initially,
conventional PCR was used, achieving detection rates ranging
from 25-30% [8,10,19]. Subsequently, a nested PCR was started
[8,22], thus achieving detection rates of 54%. It is described that it
is 10 times more sensitive than conventional PCR [22].
Other study [23] compared six PCR protocols that
use the
Thymidine Kinase protein gene for the identification of FHV-1
and analyzed its viral detection rate against a study population
affected by the virus [11]. In conclusion, it can be concluded that
the PCR that obtained the best results with a viral detection rate of
89 % [22], which included a nested PCR (n-PCR). say, he used two
pairs of different primers in sequential amplification reactions.
The first pair of primers amplifies a DNA fragment which is then
used as a template in a second reaction. The pair of primers used
in the second round of amplification, verifies the specificity of
the product obtained in the first PCR and the transfer of the first
product obtained to a new reaction mixture, has the useful effect
of dilution of the possible inhibitors that may exist in the original
sample [4]. Thus, the work of Stiles and colleagues is the main
reference in this report to achieve the implementation of a PCR (in
this case nested) that seeks to detect the Thymidine Kinase gene
from feline herpes virus, to complement the clinical diagnosis of
the virus, since there is currently no molecular diagnostic test for
this specific virus in Chile.
Material and Methods
Samples. Samples from 11 short-haired domestic cats under
one year of age were used, without previous vaccinations, who
presented the clinical signs of: conjunctivitis uni or bilateral,
mucopurulent nasal and ocular discharge, paroxysmal sneezing,
blepharospasm and/or corneal ulcer, typical of acute infection
with FHV-1. Subsequently, each of them underwent a swab with
a dry sterile tórula on the ventral conjunctival mucosa of one or
both affected eyes. The samples were kept refrigerated at 4 °C for
3 weeks, before the content of the swab was homogenized in a
tube with 200uL of nucleated free water (Winkler®), previously
numbered, using a Heidolph® tube agitator. The Feligen®
lyophilized triple-feline attenuated vaccine from the Virbac
Laboratory was used as a positive control, which was resuspended
in 500 uL of the diluent recommended by the manufacturer. As a
negative control, a swab with a sterile swab was obtained from
each eye of a cat clinically free of feline herpes since childhood and
without vaccinations.
Detection of the Thymidine Kinase (TK) gene in FHV- 1 by n-PCR
Nested PCR
The primers used to detect the gene Thymidine Kinase of
FHV-1 in this first reaction (PCR1) amplify a fragment of 383 base
pairs (bp) (Reubel et al., 1993)) and were commissioned to the
company Bioscan® for its preparation: FHV-1A 5’ –GCATTTACATA
GATGGTGCCT – 3’ and 5’ –ATATCTTGCG AGTGGGAAACAG – 3’. In
the second reaction (PCR2), a second pair of primers was used to
amplify an internal segment of DNA obtained in PCR1 and with a
size of 224 pairs of DNA. bases (Stiles et al., 1997): FHV-1B 35’ –
CTTAC TACTTCCCAGAACC – 3’ and 5’ – GTTCC T CACATACAACTTTC
– 3’.
Reaction Mixture
15uL of the 2X PCR master mix commercial kit (Taq DNA
polymerase, MgCl2 and the deoxyribonucleotides trifostatos),
1uL of sample and 5uL of each specific splitter, in a final volume of
26uL were used. b) DNA amplification [22]: Both the first and the
second reaction of the nested PCR (PCR1 and PCR2) are governed
by the same amplification protocol: after the initial denaturation
at 94 °C for 4 minutes, a PCR sequence of 35 cycles (denaturation:
94 °C for 1 minute, alignment: 55 °C for 1 minute, extension: 72 °C
for 1 minute). Subsequently, a final elongation stage at 72 °C for 8
minutes.
Visualization of Amplified Products
It was performed by electrophoresis in 2% agarose gel (Winkler
®) in Tris-borate buffer (90 Mm Tris-borate, 10mM EDTA) as
solvent. An aliquot of 5μL of this mixture was deposited in the
respective well of the gel. Electrophoresis was carried out at 90 V
for 45 minutes. As a molecular size marker, a standard containing
DNA fragments between 100 and 1000 bp (Fermentas®) was
used. After electrophoresis, the gel was incubated in ethidium
bromide (0.5μg / mL) (Fermelo®) for 45 minutes and then placed
in a transilluminator of ultraviolet light (Transiluminator UVP ®)
and photographed.
Biosafety Measures
The laboratory work was carried out in accordance with the
biosafety levels established for microbiology and animal virology
laboratories, such as the use of clean material, correct waste
disposal and the use of a closed apron, and gloves in practical
work. The process of visualizing the amplified product involves
the use of ethidium bromide and a UV light transilluminator. Due
to this, at the time of visualizing the gel an acrylic plate and glasses
with UV filter were used. Subsequently, the elimination of the gel
submerged in ethidium bromide contemplated its incineration,
since the chemical compound mentioned has -among othersmutagenic
properties.
Determination of Percentage of Nucleotide Identity with Respect to Data from Genbank®. Sequencing
After being submitted to the first PCR, two samples were sent
to the Sequencing Center of the company Genytec Ltd. according
to their requirements, which performed the purification of the
amplified. The sequences were made using the Big Dye Terminator
Kit, Applied Biosystems, and an ABI PRISM 310 computer was
used to read them. Genetic Analyzer (Genytec specifications).
Analysis
Using the open-access online program ClustalΩ, the sequences
delivered by Genytec Ltd. were aligned to achieve a consensus
sequence (PMS) that was then compared with the fragment of
the Thymidine Kinase from the feline herpes virus of GenBank®
(access number E12463 .1), thus establishing the percentage of
nucleoid identity. By way of comparison, the results of Genytec Ltd.
were also incorporated into the BLAST Online Access Program.
Results
Nested PCR
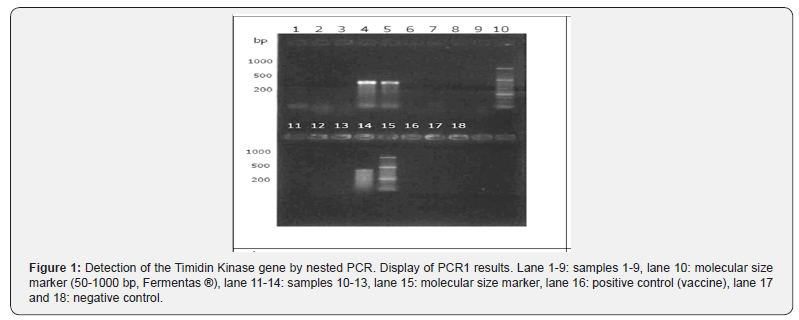
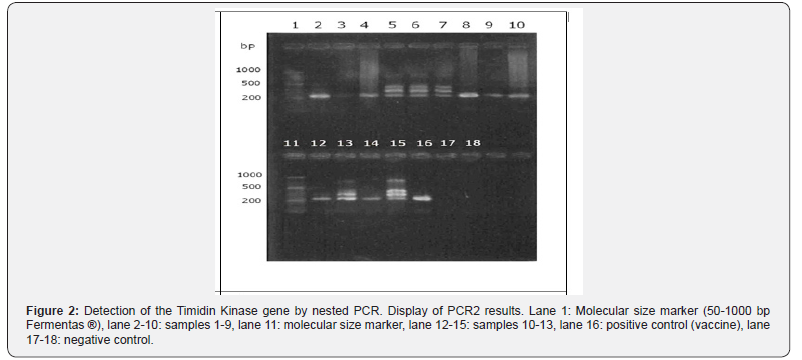
When performing PCR1 (Figure 1) it was observed that in 3 of
the 13 samples a DNA fragment of a size close to 400 base pairs
was visualized, achieving a visible band in the clear and wide gel.
However, in the positive control (vaccine) a visible band was not
obtained. On the other hand, in the PCR2 products (Figure 2) it
was possible to observe in all the samples and positive control
a fragment of DNA of molecular size around 200 base pairs,
achieving a band of different quality of display. These samples can
be diagnosed as positive.
Analysis of the Sequenced Fragment
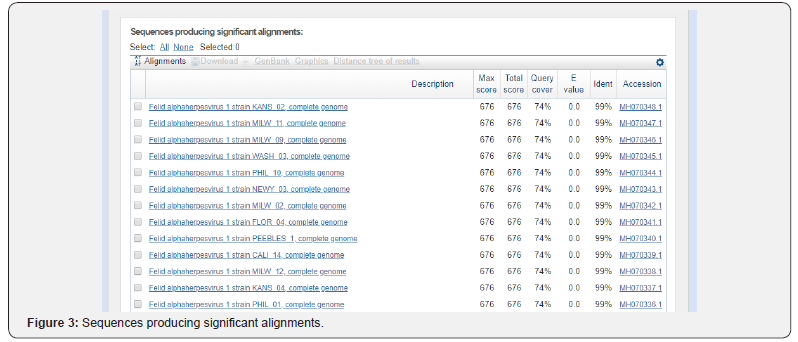
The obtained amplifications were successfully sequenced.
Subsequently, they were aligned to obtain a consensus sequence
(PMS) and thus compare with with the GenBank® data for FHV-
1 using the BLAST program (Figure 2 & 3), obtaining an average
value of 99% nucleotide identity.
Discussion
Based on the results obtained in this study, the following
reflections can be made: in PCR2 all samples were positive, so
it is thought that there was indeed amplification when using
primers 1 and 2 in PCR1, but because after PCR1 the samples and
the positive control had a low number of amplicons, they did not
generate a visible band in the UV transilluminator. The results of
the PCR2 are explained by the condition of being a nested PCR,
where in the second reaction it was sought to amplify a segment
immersed in the amplicon resulting from the first PCR, increasing
the sensitivity and specificity of the test.
The positive samples in PCR1 corresponded to individuals who
had severe clinical signs, with blepharospasm in all and epistaxis
in sample 5, which suggests that the greater the severity of clinical
signs, the greater the amount of virus excreted, which entails to
an evident visualization of the band in the UV transilluminator. In
support of this premise, in the PCR2 results it was observed that
sample 1 and 2 (belonging to the same cat) were positive, but with
differences in their sharpness. The band visualized in sample 1
was evidently more extensive and marked than sample 2, which
was less visible. This was obtained from the right eye, which
presented greater clinical severity with respect to the left eye,
from which sample number 2 was obtained. This same situation is
repeated in samples 11 and 12, but of a different cat. This explains
that with 11 cats under study, 13 samples were obtained.
In PCR2, it was possible to appreciate that the primers used are
specific, since in 8 out of 13 samples they generated a single band
of 224 bp. It should be noted that these 8 samples did not present
visible bands after visualization in the UV transilluminator after
PCR1. In contrast, in the remaining 5 samples, 3 bands of different
molecular size were observed, which, based on the molecular
marker, would be approximately 400, 300 and 200 bp. Bands of
400 and 200 bp would correspond to the amplicon obtained in
PCR1 (383 bp) and that obtained in the PCR2 (224 bp) respectively.
The appearance of a third band of 300 bp could be explained
because the samples that were used for PCR2 corresponded to the
resulting solution after PCR1, causing that in the second reaction
were the four used starters, which are governed under the same
temperature protocol in the thermal cycler, that is, they are
specifically linked to a mold thread at the same time, generating
four possibilities of amplicons depending on their association. The
combinations of starters according to the direction of nucleotide
synthesis (from 5 ‘to 3 ‘) would be: 1-2 (383 bp), 3-4 (224 bp), 1-4
(311 bp) and 2-3 (295 bp). Since the last two would have a similar
molecular size, it is explained that in the UV transilluminator only
3 bands are appreciated instead of 4.
The five samples in which the 3 previously described bands
were observed (3 of which were the only positive ones in the
PCR1), correspond to the cats that were more severe in their
clinical signs comparatively with the rest of the individuals in the
study. If these cats would excrete a greater amount of virus, the
explanation for the appearance of the 3 bands only in these cases
could be because having a large amount of viral DNA in a sample
and then carrying out the PCR, visualize all the amplicons formed.
On the other hand, when there is scarce viral DNA, only the most
amplified segment in the UV transilluminator can be observed,
corresponding to that obtained thanks to primers 3 and 4, added
in the second PCR reaction. For this reason, it is proposed that in
those samples when the nested PCR is visualized these 3 bands,
repeat the PCR2, considering that instead of using 1uL of sample,
take 0.1 - 0.5uL.
It is important to point out that this phenomenon is not
observed in any of the samples of the publication that is taken as
reference in this report [22]. A possible explanation could be due
to the characteristics of the cats sampled in both studies, since at
present only cats under one year were selected. On the other hand,
Stiles et al. [22] considered felines of all ages, mainly adults, whose
clinical picture was characterized by lower severity, and therefore
lower viral excretion. In relation to the high detection rate, it is
important to note that 9 of the cats sampled (9 out of 11) belong
to the same feline refuge, where they have shared for 2 weeks a
small cage. Knowing the high morbidity of FHV-1 there is a high
possibility that all of them are infected.
In this report it could also be determined that, consequently
with the results obtained in a recent study [23], three weeks of
refrigeration did not influence the detection of FHV-1 by PCR,
given that all the analyzed samples were positive. Compared with
other PCR protocols worldwide, the implemented in this work
reached a high detection rate. A study conducted at the University
of California in 2005 [23], compared the “performance” (relative
detection rate, and the minimum detected by dilutions) of 6 PCR
protocols described by different researchers who have in common
to detect the gene of the Thymidine Kinase, which were the most
used in laboratories. They differ in the specific segment to be
amplified from the gene, the type of PCR (conventional or nested),
the starters, temperature and number of cycles in the thermal
cycler. The results show detection rates from 29 to 86%. It should
be noted that the samples corresponded to cats suspected of FHV-
1 and it was not verified whether they were indeed infected by
other diagnostic tests, such as viral isolation.
Among the protocols that entered the study (Reubel et
al.,
1993) that uses the same PCR protocol and PCR1 primers of this
title memory, but performs only a conventional PCR, obtaining a
29% rate of detection. In another study [22], another successive
PCR with different primers was added to the Reubel conventional
PCR, that is, it performed a nested PCR, obtaining a rate of 54%
detection. Years later, it was replicated by another researcher [11]
who obtained 86%, in contrast to the present study that obtained
a 100% detection rate. The cats that entered the different studies
[22-24] had a wide range of age (7 months to 15 years) and were
suspected of infection with FHV-1, both acute and chronic. These
variables are the ones that would explain that, despite being the
same nested PCR protocol, different detection rates are obtained.
That is why in this title report we sought to minimize these
variables, focusing on the detection rate in individuals under one
year of acute infection with FHV-1, therefore, it is proposed in
later studies to detect this virus in adult cats that are studying the
chronic phase of the disease, with the signology that is associated
with such clinical picture. The high percentage of nucleotide
identity obtained shows that indeed the amplified DNA fragment
corresponds to the gene of the protein Thymidine Kinase of the
feline herpes virus, and not to another herpes virus, although it
is known that one of the characteristics of the viral Alpha herpes
virus family is to be highly species-specific.
Conclusion
In this report, the molecular diagnosis of FHV-1 is first
described in Chile by the detection of the Timidin Kinase protein
gene by the nested PCR technique, which has a high detection rate
(100%) in cats. infected children under one year of age, who are
in the acute stage. The percentage of nucleotide identity obtained
of 92% allows to affirm that the detected virus corresponds to the
feline herpes virus.
To know more about journal of veterinary science impact factor: https://juniperpublishers.com/jdvs/index.php
To know more about Open Access Publishers: Juniper Publishers




Comments
Post a Comment