Effect of Selenium and Vitamin E on Development and Viability of Preimplanted Mouse Embryo- Juniper Publishers
Journal of Dairy & Veterinary Sciences- Juniper Publishers
Abstract
In vitro culture results higher level of
reactive oxygen species (ROS) oxygen than in vivo environments that
cause lipid peroxidation of cellular membranes. Selenium (Se) and
Vitamin E (Vit-E) are the important antioxidants that protect mammalian
cells against lipid peroxidation. Therefore, the present study was
conducted to investigate whether Se or Vit-E and Se+Vit-E overcome the
the undesirable oxidative stress produced by hydrogen peroxide (H2O2)
and enhance the development of pre implanted mouse embryo.
Co-incubating the embryos with 60nM Se and/or 100nM Vit-E were increased
(P<0.05) the blastocyst development rate.
The addition of H2O2 reduced the development of mouse embryo, but the addition of Vit-E, Se and Se+Vit-E reduced the detrimental effect of H2O2
and influenced the higher rate of development to blastocysts, compared
to CZB alone (P<0.05). The incorporation and oxidation of 14C-glucose
in the blastocysts developed by the medium supplemented with Se and/or
Vit-E in the presence or absence of H2O2 were
significantly higher (P<0.05) than that of the control. Moreover,
Vit-E is more effective than Se and Se+Vit-E in reversing ROS-induced
mouse embryo toxicity. Therefore, Vit-E may be supplemented in the CZB
medium for better development and viability of pre implanted mouse
embryo.
Keywords: Mouse embryo; Selenium; Vitamin EAbbrevations Ros: Reactive Oxygen Species; -Oh: Hydroxyl Radical; O2-: Superoxide Anion Radical; Se: Selenium; Gpx: Glutathione Peroxidase; Cpm :Counts Per Minutes
Introduction
In vitro embryo culture suffers from excessive
developmental failure. Its inefficiency is linked with the generation of
reactive oxygen species (ROS), such as H2O2, hydroxyl radical (-OH) and superoxide anion radical (O2-), appears as the by-products of cell metabolism [1].
Superoxide may also spontaneously break down into oxygen and H2O2. As
ROS are highly reactive molecules, their accumulation can lead to damage
and breakage of DNA strands. There are many evidences have been found
that ROS compromises embryo development in many species [2-6].
Selenium (Se), an essential trace element for mammals, is an integral part of anti- oxidant system [7].
Se dependent glutathione peroxidase (GPx) has an important role in free
radical protective mechanisms. Vitamin E (Vit-E), the predominant
lipid-soluble antioxidant in animal cells, protects cells from oxygen
radical damage in vivo [8,9] and in vitro [10,11].Our
previous study investigated that Se and Vit-E, as the integral parts of
antioxidant systems which play important roles for the in vitro
maturation, fertilization and culture of porcine oocyte [12].
However, there is a very limited studies were conducted with the effect
of Se and Vit-E on the development of preimplanted mouse embryo.
Therefore, the present study was conducted to investigate whether Se,
Vit-E or Se+Vit-Eovercome the undesirable oxidative stress produced by
hydrogen peroxide (H2O2) and enhance the development of preimplanted mouse embryo.
Materials and Methods
Chemicals and reagents
The basic embryo culture medium used in this study was CZB [13] , which contains 1mM glutamine, 0.1 mM EDTA and 5mg/ml BSA, and considered as control. A part of CZB was added with 30% H2O2
to get a final concentration of 0.0003% that considered as negative
control. According to the experimental layout, CZB (with or without H2O2)
was supplemented with30, 60, 90Nm Se (sodium selenite; Sigma-Aldrich,
St. Louis, MO, USA), 50, 100 and150nM of Vit-E (α-tocopherolacetate) and
their combination (Se+Vit-E). Selenium, Vit-E and H2O2 were equilibrated in culture medium under CO2
incubator for 8 hours before the start of culture. Vit-E was dissolved
in ethanol, and an emulsion was formed by vortex mixing before adding it
to the embryo. PMSG and HCG used in this study were obtained from
Sankyo Chemical Industries Ltd., Tokyo, Japan. The copulation plug was
checked 24 hours later. Radioactive 14C (U)-glucose was purchased from
American Radio labeled Chemicals (St. Louis, Mo, USA). All other
chemicals were of analytical grade and purchased from Nacalai Tesque
(Kyoto, Japan) unless otherwise indicated.
Collection of embryos
Embryos were obtained from 7-8 weeks old female ICR
mice. They were offered feed with a balanced standard diet and ad
libitum clean drinking water. Animals were kept in polycarbonate cage
with wood shavings under a 12h light: 12h dark regimen (light on at
6:00), at a temperature of 20±1 °C in accordance with the "Guideline for
Regulation of Animal Experimentation, Faculty of Agriculture, Shinshu
University.” Female mice were induced to superovulation with PMSG (5 1U,
i.p.) followed 48h later by hCG (51U, i.p.) and met with male mice.
Zygotes of 1-cell stages were collected at 2 5 hours HCG post injection
by flushing out from the fallopian tubes. The embryos were subsequently
incubated in CZB medium.
Experimental layout
In fact, there are four experiments were conducted
under this study In experiment 1, effect of CZB supplanted with
different levels of Se (0, 30, 60 and 90nM) and Vit-E (0, 50, 100 and
150nM) on the development of mouse embryo from 1-cell stage to the
blastocyst were evaluated. The experiment 11 were conducted to
investigate the effects of Se (60nM), Vit-E (100nM) and their
combination (60nM Se+100nM Vit-E) on the development from 2-cell to
blastocyst stages of embryo in the presence (1mM) or absence of H2O2
in CZB medium. Where, the experiment 111 was conducted to the effect
evaluate Se (60nM), Vit-E (100nM) and their combination (60nM Se+100nM
Vit-E) on the accumulation of ammonia (NH4) in presence or absence of H2O2
due to metabolism of embryo during the development up to blastocyst
stages. 1ncorporation and oxidation of 14C-glucose at blastocysts
developed by the supplementation of Se (60nM), Vit-E (100nM) and their
combination (60nM Se+100nM Vit-E) in the presence or absence of H2O2
were evaluated by conducting the experiment IV. These experiments were
conducted five times repeated.
Culture of embryos
The collected zygotes of 1-cell stages were
transferred to the culture dishes for washing, and grown in-vitro to the
developmental stages up to blastocyst. Embryos were cultured according
to the standard techniques, in groups of 10 zygotes were placed into
35mm-diameter culture dishes (Nunc Co., Denmark) containing 30|il of
each CZB medium under a layer of paraffin oil and equilibrated overnight
in an atmosphere of 5% CO2 in air at 37 °C. The pH of all
media was 7.4 after equilibration. The developing embryos seemed to be
normal in their morphology, with almost no fragmentation.
Ammonia determination
During incubation period embryos which developed in the presence of Se and/or Vit-E with or without H2O2,
the ammonia concentrations in the medium were assessed by using the
Bertholot-indophenol method as described in our previous study [14]
. To determine the ammonia concentration in the medium, 100μ1 of the
culture medium was removed every 2-4h and frozen at -40 °C until
measurement. The procedure was carried out five times for the analysis. A
calibration curve in the range 0.0003� of ammonia was run with each
experiment. The mean coefficient for determination of the calibration
curve of five experiments was 0.994.
Incorporation and oxidation of 14C-glucose
The experiment was initiated with 14C-glucose
18.5kBq/0.1mol (specific activity 9.69MkBq/mol, Moravek Biochemicals,
1nc., USA). Each of the ten blastocysts which developed in the presence
of Se and/ or Vit-E with or without H2O2 was
transferred in a microtube of 50μ1 CZB medium drop containing
14C-glucose then overlaid with mineral oil. On the other hand, 1ml of
2.5Mm NaOH solution was transferred into a 1.5ml micro tube as a trap
for the evolved 14 CO2. Both microtubes of NaOH and
14C-glucosewith embryos were confined into a scintillation vial using a
rubber stopper. The scintillation vials were incubated for 5h in an
incubator at 37 °C. After incubation period, the metabolic reactions of
embryos were stopped with an injection of 100μ1 of 10% perchloric acid
(PCA) kept at room temperature for 24h. The acid insoluble materials
were carefully washed by millipore filtration (8.0|iM white SCWP, 47mm;
Millipore Corporation, Bedford, MA, USA) with 5% PCA and the filter
papers were kept overnight under a lamp. After drying, the filter papers
were transferred into scintillation vials. The NaOH solution was
transferred into a new scintillation vial by washing 3-4 times with
cocktail (0.5% PPO+0.03% POPOP solution in toluene). All the
scintillation vials with 5ml of cocktail were set in a Liquid
Scintillation Counter (LS-6500, Beckman Instruments, Inc. USA) to
determine the levels of radio activity [15].
This experiment was conducted ten times to improve its accuracy. The
values of incorporation and oxidation were expressed directly as counts
per minutes (CPM).
Statistical analysis
Data obtained from this stud were analyzed by one-way
ANOVA using the GLM procedure of SAS (SAS Institute, Cary, NC). The
data expressed as percentage were tested by Chi-square test. Data were
presented as mean ±SEM of at least 5 replicates and differences were
considered significant at the level of P<0.05.
Results
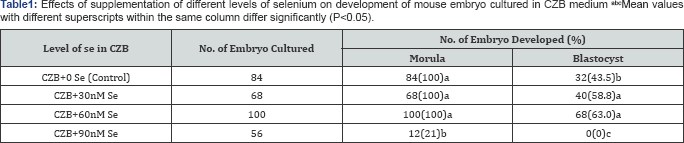
The effects of supplementation of different levels of
Se (0, 30, 60 and 90nM) in CZB medium on the development of mouse
embryo are presented in (Table 1).
The results revealed that 30 and 60nM of Se was more effective
(P<0.05) for mouse embryo development than that of the control and
90nM of Se, where the level showed detrimental effect on the development
of embryo as blastocyst. On the other hand, (Table 2)
demonstrated that the supplementation of 100nM of Vit-E was the most
effective (P<0.05) for embryonic development than that of the 0,
(control), 50 and 150nM. The lowest (P<0.05) percentage of blastocyst
was observed when zygotes were cultured in CZB supplemented with 150nM
of Vit-E.

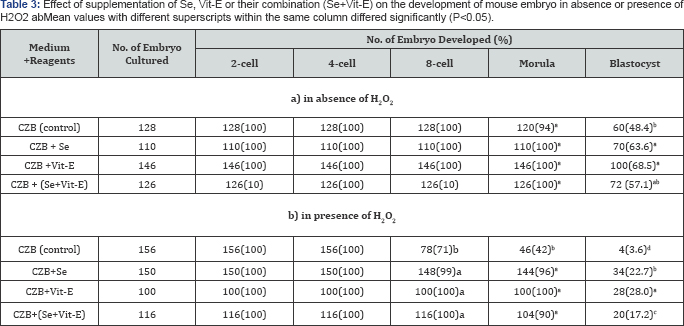
Detrimental effects of H2O2 and reducing or protecting ability of Se, Vit-E or their combination from the effects of H2O2 on the embryonic development were shown in (Table 3). The results demonstrated that the development of mouse embryos was reduced by the detrimental effect of H2O2 especially in CZB, the occurrence after 8 cell markedly decreased under H2O2 (P<0.05). However, the supplementation of Se, Vit-E and Se+Vit-E were able to reduce the detrimental effect of H2O2
and enhanced (P<0.05) the development of mouse embryo to be
blastocyst. Highest percentage of blastocysts was obtained when the
zygotes were cultured in the CZB medium supplemented with Vit-E in the
presence or absence of H2O2.
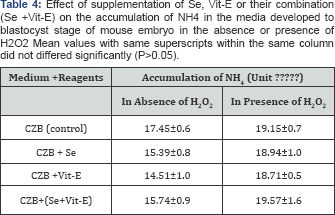
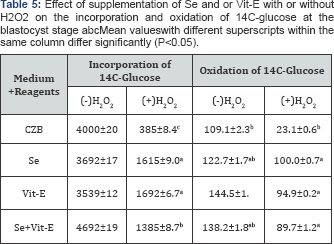
During the development of mouse embryos up to the blastocyst stage, accumulation of metabolic NH4+ in the CZB medium supplemented with Se, Vit-E and Se+Vit-E in the presence or absence of H2O2 is showed in (Table 4). The results revealed that the lowest (P<0.05) accumulation of metabolic NH4+ was observed in the CZB medium supplemented with Vit-E in the presence or absence of H2O2. The effects of supplementation of Se, V1t-E and Se+Vit-E in the CZB medium with or without H2O2 on incorporation and oxidation of 14C-glucose at the blastocyst stage are shown in (Table 5).
The incorporation of 14C-glucose at the blastocyst stage cultured with
Se, Vit-E and Se+Vit-E in the CZB medium were not significantly differed
(P>0.05), but higher rate of incorporation (P<0.05) occurred by
the supplementation of Se or Vit-E in the presence of H2O2. On the other hand, in the presence of H2O2,
the oxidation of 14C-glucose by the blastocysts were higher (P<0.05)
when zygotes were cultured in the CZB medium supplanted with Vit-E or
Se alone or Se+Vit-E than the basic CZB medium. There was a slightly
higher rate of oxidation of 14C-glucoseoccured in the blastocyst stage
cultured with Se, Vit-E and Se+Vit-E.
Discussion
A number of intrinsic and extrinsic factors have been
shown to influence in vitro survival of the embryos to the blastocyst
stage in extended culture. Previous studies suggest that in vitro
extrinsic factors such as prolonged culture conditions and the
autocrine and paracrine activities of the embryos may also contribute to
the failure of optimal embryo development. Among the factors that might
affect in vitro development of embryos is the balance between oxidative stress, and the ability of the embryos to neutralize their effects [16]
reported a sustained increase in oxygen, glucose and pyruvate uptake
during in vitro embryo development. The embryos were dependent on
oxidative phosphorylation for energy (ATP) production at all stages of
pre-elongation development, with perhaps a shift in dependence towards
glycolysis in conjunction with compaction. This enhancement in oxidative
metabolism of the embryo could be linked to the detected increase in
ROS, which are characterized by the presence of an unpaired electron [17] and free-radical intermediaries [18].
Free radicals are generated from leakage of
high-energy electrons as they proceed down the electron transport chain.
The free radicals have many harmful effects including DNA damage [19].
Many embryos under oxidative stress step into a transient cell cycle
arrest which is activated by DNA damage response before apoptosis [20]. Legge & Sellens [21]
were suggested that the 2-cell block in mouse embryo is at least in
part, of free radical damage incurred by embryos during collection and
culture, and that medium supplementation with the radical scavenger,
reduced glutathione, can improve embryo development in vitro reported
that enhanced oxidative metabolism of embryos may be associated with
increased ROS levels detected. The gradual increase in ROS levels from
the 2-cell embryo up to the late morula stage could depend on the
metabolic change undergone by the embryo during its development. 1t is
necessary to prevent ROS as much as possible during culture embryos.
However, it is unclear as to which embryos may be adversely affected and
to what extent.
The present study showed that Se, Vit-E and SE+Vit-E
increased blastocyst formation compared to control. Especially, it
suggested that the formation of mouse blastocysts cultured in the
presence of 60nM/ml Se and 100nM/ml Vit-E were significantly higher than
control. The trace element Se is a component of antioxidative seleno
enzymes, Glutathione Peroxidase (GPx) and ThioredoxinReductase (ThxRed)
that decrease oxidative stress. Se, as sodium selenite, has been
reported as a co-factor for glutathione peroxidase and other proteins
and used as an anti-oxidant in medium [22].
1n cell culture system, sodium selenite protected cell from oxidative
damage, free radicals and obstructed lipid peroxide products [23,24].
Se played a role in the antioxidant defense system in the formation of
mouse blastocyst, which was essential for the catalytic activity of
glutathione peroxidase. Glutathione, a thiol tripeptide component in all
cell types has an important role in the transportation of amino acid,
synthesis of the protein and DNA, and reduction of disulfide bonds [25].
In the present study, Se and Vit-E were used as
combined supplements in the CZB medium for culture of mouse embryos, but
this combination showed lower influence in the development of mouse
blastocyst than Vit-E alone. Alpha-tocopherol (Vitamin E) is well known
as an ROS scavenger in in vivo and in vitro conditions [26-28]
and is the most important antioxidant present in ovarian tissue and
follicular fluid. The antioxidant activity of a-tocopherol in preventing
free-radical-induced tissue damage is accepted by most investigators
and is believed to be the primary free radical scavenger and to inhibit
lipid peroxidation in the mammalian cell membrane [26-29].The
present study also demonstrated that the supplementation of Vit-E
(specially, 100nM) played an important role in the development of mouse
embryo. The results are in agreement with the results of our previous
study and the study of [30] they also reported that the optimal concentration of Vit-E in embryo culture is 100nM.
There is a method to observe the effect of mild
oxidative stress with retardation of embryo development in the medium
supplemented with 1-5mM H2O2. The H2O2 used in this experiment was 1mM, and performed detrimental effects on the development embryo after 8 cells [31]. Reported that effects of H2O2
on blastocyst formation became more severe during the treatment of
later stages of development. Embryos may also have different
sensitivities to ROS at different developmental stages [32]. The exogenous oxidant H2O2
leads to over production of ROS, which may induce multiple cellular
damages, including lipid peroxidation, nuclear DNA strand breaks, and
mitochondrial alteration, consequently disturbing the development of pr
implanted embryos in vitro [33-35].
Most of the embryos under oxidative stress step into a transient cell
cycle arrest in vitro, which is activated by DNA damage response before
apoptosis.
Vit-E(α-tocopherol)isapredominantlipid-solubleantioxidant
that has been considered as a primary free radical scavenger in
biological membranes [36-38].
a-tocopherolscavenges peroxyl radicals from polyunsaturated fatty acid
in membrane phospholipids or lipoproteins that do not spread the radical
chain, thereby protecting against lipid peroxidation [39].
Our previous study reported that supplementation of a-tocopherol
maintains the development of mouse embryo and pig oocyte quality,
fertilization rates and embryo development. Mouse preimplantation
embryos can be cultured in a simple defined medium. Under such
conditions energy substrates in the medium represent a major source of
carbon for anabolism. Glucose is incorporated into macromolecules during
in-vitro culture of cleaving mouse embryos and blastocysts [40].
1n particular, both acid-soluble glycogen and desmoglycogen are rapidly
synthesized from glucose presumably to act as a source of energy at
implantation [41].
Overall metabolism, as assessed by oxygen consumption is low during the
cleavage stages of development before rising sharply at the blastocyst
stage. Moreover, metabolic activity has been shown to relate to
developmental potential.
In this study, the accumulation of ammonia in the culture was measured regardless of the presence or absence of H2O2,
the lower accumulation of ammonia in the blastocyst stage is better
than the higher accumulation that occur incidence to the blastocyst [42] showed that high levels of ROS in culture media are associated with low rates of embryo development and blastocyst formation [43]. Suggested two possible mechanisms for the inhibitory effects of NH4+. Perturbation of intracellular pH requires the involvement of Na/K ATPase to transport NH4+
across membranes. Alternatively, may interact directly with enzymes,
participating in a series of futile cycles which detoxify NH4+ and result in consumption of ATP. Thus, by whichever mechanism, inclusion of NH4+in culture media will divert ATP from growth to maintenance.
Azizimoghadam reported that pentose phosphate pathway
activity of total glucose metabolism was increased at the compacted
morula stage and was highest at the blastocyst stage [44].
1n this study, incorporation and oxidation of 14C-glucose in the
blastocyst was significantly influenced when cultured with Vit-E and Se
in the presence of H2O2. The incorporation and
oxidation of 14C-glucose at the blastocyst tended to resemble the
development of embryo. The present study appeared that incorporation and
oxidation of 14C- glucose was good for showing the activity of embryos.
Generation of ROS induced by glucose utilization was
assumed to be caused by the activation of NADPH oxidase, an enzyme that
catalyzes the oxidation of NADPH, generates NADP that serves as a
coenzyme of the oxidative arm of the pentose phosphate pathway (PPP) [45].
The gradual increase in ROS levels from the 2-cell embryo up to the
late morula stage could depend on the metabolic change undergone by the
embryo during its development. 1t is necessary to prevent ROS as much as
possible during culture embryos.
Conclusion
In conclusion, our results showed that culture of
mouse zygotes in the CZB medium supplemented with 60nM Se and/ or 100nM
Vit-E improves the developmental rate of mouse blastocyst formation in
the absence or presence of free radicals or their sources. Therefore,
the present study reveals that selenium and vitamin E improves mice
blastocyst viability by minimizing the level of free radicals that might
occur during development of blastocyst in vitro and which may be useful
for assisted reproductive techniques.
To know more about journal of veterinary science impact factor: https://juniperpublishers.com/jdvs/index.php




Comments
Post a Comment