Effect of Prostaglandin F2α on Growth of Streptococcus uberis associated with Bovine Mastitis -Juniper Publishers
Journal of Dairy & Veterinary Sciences- Juniper Publishers

Introduction
The spread of mastitis pathogens causes large
economic losses to the dairy industry. These losses include increased
involuntary culling rate, reduced milk production, increase domatic cell
count, and discarded milk [1,2]. Costs associated with mastitis infections in the U.S. dairy industry have been estimated at nearly US $2 billion per year [3]. Streptococcus uberis
is one of the most common gram-positive causes of clinical mastitis and
contributes to a large proportion of subclinical mastitis cases [4,5]. Streptococcus uberis infections are often difficult to cure with traditional intra-mammary antibiotic preparations, especially in older animals [6,7]. Similar to treatment trials focused on Staphylococcus aureus (S. aureus) intra-mammary infection, increased somatic cell counts before treatment was associated with a decreased probability of cure [6]. This may explain why the response of Strep. uberis mastitis to treatment can be poor, even after extended therapy [7]. If cure rates are low, it is generally not considered cost-effective to treat cows with chronic cases infections [8].
The bactericidal activities of various fatty acids as an alternative to antibiotics have been studied and reviewed [9,10]. Kelsey et al. [11]
demonstrated that lauric acid, capric acid, myristic acid and linoleic
acid inhibited growth of two different mastitis strains of S. aureus. Arachidonic acid, a fatty acid derived from linoleic acid [12] inhibited gram-positive bacteria such as S. aureus and S. pyogenes [13]. Considering that both linoleic and arachidonic acid have been shown to affect bacterial growth, we speculated that PGF2α,
synthesized from these fatty acids, may have similar antibacterial
properties. In fact, the results from a recent study in our laboratory
[14, 15]; indicate that PGF2α, inhibits the growth of S. aureus and Mycoplasma bovis. Given that Strep. uberis is one of the most common gram-positive causes of clinical mastitis, our hypothesis was that PGF2α would inhibit the growth of Strep. uberis. The objective of this study was to determine the effect of PGF2α on Strep. uberis in vitro by characterizing the growth response of Strep. uberis to PGF2α in the form of dinoprosttromethamine.
Materials and Methods
Experimental design and treatment
Bacterial cultures were prepared by inoculating a
single colony into 3ml of tryptic soy broth (TSB) (EMD Chemicals Inc.,
Darmstadt, NJ) followed by overnight incubation at 37 °C with shaking at
250rpm. In order to obtain a sufficient culture for the experiment,
culture tubes containing 10ml of fresh TSB were inoculated at 1:100 with
3 ml of overnight Strep. uberis culture, and once more incubated overnight at 37 °C with shaking at 250rpm. Prostaglandin F2α
in the form of dinoprosttromethamine (Zoetis, Florham Park, NJ) was
added to flasks for a final concentration of 0, 0.6, 1.2, 2.4 and 4.8
mg/ml (2 flasks/treatment). Flasks, which included both treatment and
controls, were inoculated with the 10 ml overnight culture of Strep. uberis
at a concentration of 1:100. Flasks were incubated at 37 °C and shaken
at 250rpm for 24h; at 0h, and every 4h thereafter, 1ml samples were
taken from each flask to determine bacterial growth. The entire
experiment was repeated three times, in duplicate, in different days to
account for variation associated with a day effect, categorized as run.
Determination of bacterial growth
To determine colony forming units (CFU), samples of
0.5ml were taken from flasks for plating. Serial dilutions were
performed before samples were placed on agar plates (EMD Chemicals Inc.,
Darmstadt, NJ). The CFU counts were done in duplicate per sample from
each flask. Plates were incubated at least 12h, or until colonies were
apparent, at a constant temperature of 37 °C. The CFU counts from each
of the two agar plates were averaged for each of the corresponding
flasks.
Statistical analysis
The number of live cells, as measured by log CFU, was
determined by averaging the number of cells for the duplicate
concentrations of both plates at each 4h time point. An analysis of
variance (repeated measures) was carried out using the mixed procedure
of SAS (SAS Institute, Cary, NC) where the model included treatment,
time (repeated factor) and their interaction. To further analyze the
effect of treatments over time on the growth pattern and growth rate of Strep. uberis,
a full model dummy variable regression procedure was also performed.
The coincidence or equality of the estimated regression lines, the rate
of bacterial growth over time, and the point at which the inflection of
the growth curve occurred (an indication of maximum bacterial growth)
were determined. The estimation of the reduced models for each treatment
was carried out using PROC REG procedures of SAS, and that of the full
model was carried out using PROC GLM procedures of SAS. The fitted
reduced model for each treatment took the form of
Y = β0 + β 1x + β 2x2 + ε1
Where Y was the logarithmic value of the number of live cells (log CFU/ml), x represented time, β0 was the intercept (estimated log CFU/ml at time 0), β1 was the rate of increase for bacterial growth, β2
was the point of inflection, and ε represented the random error under
the classical regression assumptions. The adequacy of the fit was
determined by the significance of the parameter estimates (declared at P
<0.05), their corresponding magnitudes and signs, and the
examination of the estimated residuals.
Results
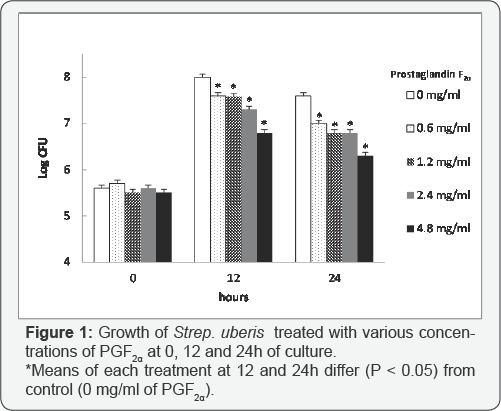
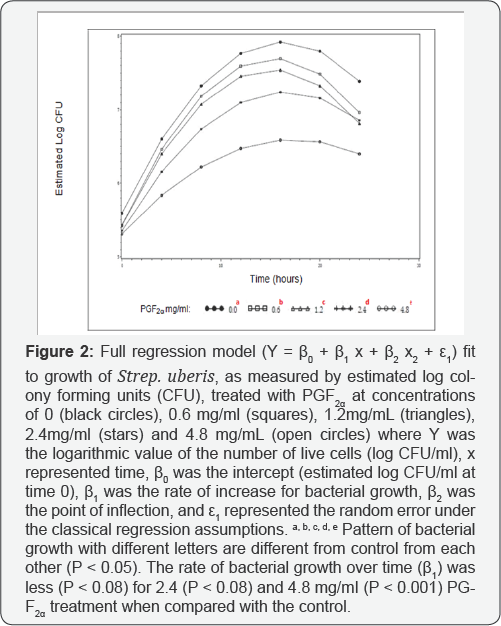
Bacterial growth curves were evaluated in growth media containing PGF2α at concentrations of 0, 0.6, 1.2, 2.4mg/ ml and 4.8, with 0 mg/ml referring to the control with no PGF2α. Based on bacterial growth curves, PGF2α, in the form of dinoprosttromethamine, has inhibitory effects on growth of Strep. uberis (Figure 1). Overall, growth of Strep. uberis decreased with increasing concentrations of PGF2α, with 4.8 mg/ml of PGF2α being the most inhibitory (Figure 2).
There was an effect of treatment and treatment by
time interaction on log CFU/ml (P < 0.05), providing evidence that
bacterial growth of Strep. uberis over time was not similar among PGF2α
treatments. Preplanned contrasts were conducted to compare the mean log
CFU/ml values between treatments at 12 and 24h. At 0h the mean log
CFU/ml values were not different among treatments and control, and
averaged 5.6±0.02 log CFU/ ml (Figure 1). Mean log CFU/ml values at 12 and 24h for each PGF2α treatment dose, however, were different (P < 0.05) from the control (Figure 1). At 12h after incubation, the bacterial growth for each PGF2α treatment reached its maximum (Figure 1).
Mean log CFU/ml for 0.6 mg/ml (7.7±0.06 log CFU/ml), 1.2 mg/ml
(7.6±0.06 log CFU/ml), 2.4 mg/ml (7.3±0.06) and 4.8 mg/ ml (6.7±0.06)
were all different (P < 0.05) from 0mg/ml (control, 8.0±0.06). At
24h, mean logs CFU/ml for 0.6 mg/ml (6.9±0.06 log CFU/ml), 1.2 mg/ml
(6.8±0.06 log CFU/ml), 2.4 mg/ml (6.8±0.06) and 4.8 mg/ml (6.4±0.06)
were also different (P < 0.05) when compared with 0 mg/ml (control,
7.5±0.06) (Figure
1) . Interestingly, PGF2a at the greatest dose (4.8 mg/ml) had the
greatest effect on bacterial growth as log CFU/ml never reached above
6.4 log CFU/ml.
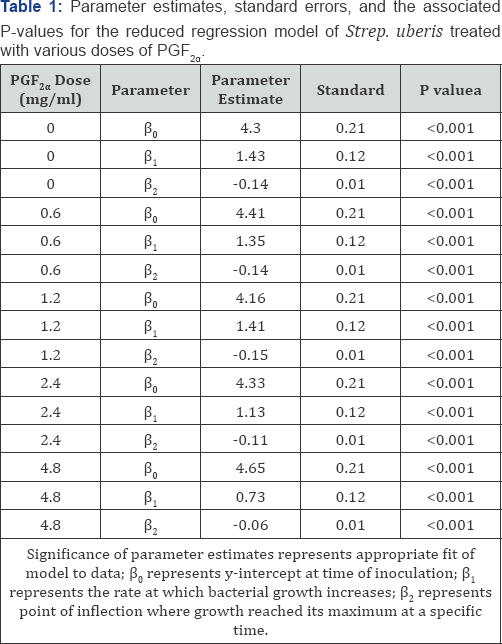
The reduced and full dummy variable models were
carried out to evaluate the effects of different PGF concentrations on
2a
the growth pattern of the bacteria concentrationson the growth pattern
of the bacteria over time. The parameter estimates of the reduced model
for all treatment doses of PGF were2 a significant (P < 0.05),
indicating that the reduced model fit the data well for each of those
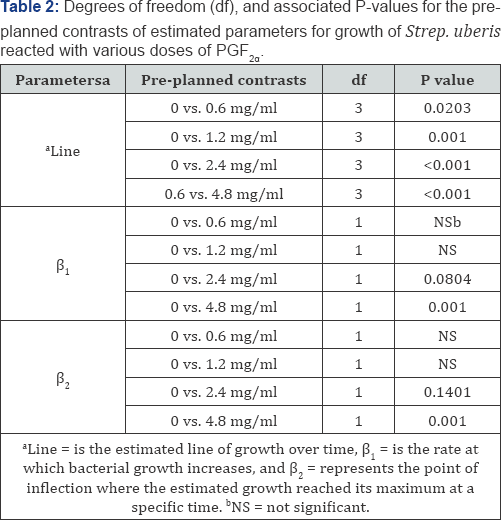
treatments and that all parameters are required (Table 1, Figure. 2). The preplanned contrasts carried out using the dummy variable regression model (Table 2)
indicated that the overall line of growth over a 24h period was
different (P<0.05) for treatments 0.6, 1.2, 2.4, and 4.8 mg/ ml, when
compared with the control (0 mg/ml), implying that the bacterial growth
pattern for these treatments were different from control and lending
support to results found through the repeated measure analysis. The rate
of bacterial growth over time (β1 was less (P < 0.08) for 2.4 (P < 0.08) and 4.8 mg/ml (P < 0.001) PGF2α
treatment when compared with the control, providing evidence that the
rate of bacterial growth over time was different between those
treatments in a dose dependent manner (Table 2). In addition, the rate of bacterial growth was slower in 4.8 than with 1.2, 2.4mg/ml PGF2α
(data not shown). Each growth curve had an estimated point of
inflection, where the estimated maximum log CFU/ml was reached at a
specific time. The estimate parameter point of inflection (P2 the estimate of maximum bacterial growth) for 4.8 mg/ml PGF2α treatment was different from the control (P < 0.05, Table 2) and all other PGF2α treatment groups (P < 0.05, data no shown).
Discussion
This research addresses the question whether the fatty acid PGF2α is inhibitory to growth of Strep. uberis. The results support
the hypothesis that PGF2α, in the form of dinoprosttromethamine has inhibitory effects on growth of Strep. uberis . These findings support the results from our previous research in which PGF2α, inhibited growth of gram positive bacteria, S. aureus [14] as well as Mycoplasma bovis [15].
The antimicrobial properties of fatty acids on bacteria have been
studied for years. The effectiveness of fatty acids in inhibiting growth
of several gram-positive bacteria have also been demonstrated and
reviewed [11,13,16].
Arachidonic acid, a fatty acid originally derived from linoleic acid, has been shown to inhibit gram-positive bacteria such as Streptococcus faecalis and Staphylococcus epidermidis, and S. aureus [13]. The authors hypothesized those bactericidal effects on S. aureus mediated by peroxidation of arachidonic acid. Because both linoleic acid and arachidonic acid are precursors to PGF2α, it is plausible that PGF2α,
synthesized from these fatty acids, has similar antibacterial
properties. The results supported our hypothesis that commercially
available PGF2o (dinoprosttromethamine) inhibited the growth of Strep. uberis in vitro in a dose dependent manner (Figure 3), resembling the actions of linoleic acid on growth of S. aureus Novel as previously described [11].
The mechanism by which PGF2α affected Strep. uberis
cannot be determined from the current study. The inhibitory properties
of fatty acids were more noticeable with increased chain length and
degree of un-saturation [10,13,17]. Zheng et al [13]
found differences in antibacterial activity between unsaturated fatty
acids and saturated fatty acids in that saturated fatty acids had less
or no antibacterial activity against S. aureus and S. pyogenes.
Dinoprosttromethamine contains two double bonds and consists of 24
carbons. These features may be important factors in its antibacterial
properties.
One potential mechanism of action centers on the
ability of fatty acids to penetrate and disruptthe phosphor lipid
bi-layer of the plasma membrane of bacteria and ultimately increases the
negative charge on the bacterial membrane surface [10]. Zheng et al. [13]
proposed that antibacterial action of unsaturated fatty acids is
mediated by inhibition of bacterial enoyl-acyl carrier protein reductase
which is an essential component of bacterial fatty acid biosynthesis.
Another proposed mechanism involves the hindering of bacterial growth
via an interaction with lipid bi-layer of the cell membrane, resulting
in a change in membrane permeability, or the interference with
transduction cascades leading to cell lysis [10, 18]. In summary, the
current in vitro results provide evidence that the fatty acid PGF2α, in the form of dinoprosttromethamine, has inhibitory effects on the growth of Strep. uberis in a dose dependent manner. The potential use of PGF2α, as an anti-bacterial fatty acid, for treatment of mastitis requires more research.
To know more about journal of veterinary science impact factor: https://juniperpublishers.com/jdvs/index.php
To know more about Open Access Publishers: Juniper Publishers



Comments
Post a Comment