Effect of Organic Acid Treated Diets Fed During Finisher Phase on Gut Microbiota and Blood Profile of Broiler Chickens-Juniper Publishers
JUNIPER PUBLISHERS-OPEN ACCESS JOURNAL OF DAIRY & VETERINARY SCIENCES
Abstract
An experiment was conducted to determine the effect of organic acid treated diets fed during finisher phase on haematology, serum chemistry, gut pH, digesta viscosity and gut microbiology of broiler chickens. The organic acids were acetic, butyric, citric and formic acids. One hundred and fifty (150) day old Abor-Acre chicks were used. There were five dietary treatments. Diet 1 which served as control contained no organic acid, while diets 2, 3, 4 and 5 respectively contained 0.25% acetic, butyric, and citric and formic acids respectively. Each treatment was replicated three times with 10 birds per replicate, arranged in completely randomized design (CRD). The experiment lasted for 4 weeks. Feed and water were given ad libitum. Mean corpuscular volume was reduced by formic acid while other hematological parameters were not significantly (P>0.05) altered. The organic acids did not alter serum parameters except aspartate amino transferase which was increased by acetic and butyric acids. The pH in the gizzard, duodenum and caecum was reduced (P<0.05) by the acids. Viscosity in duodenum was reduced by all the acids. All the acids reduced bacterial population in crop, gizzard, duodenum and caecum. It is concluded that the organic acids could be used to reduce gut bacterial population, digesta pH and viscosity.
Keywords: Broiler chickens; Haematological; Gut microbiology; Gut pH; Serum chemistry; Viscosity
Abbreviations: CRD: Completely Randomized Design; CFU: Colony Forming Units; ANOVA: Analysis of Variance; AST: Aspartate Amino Transferase
Effect of Organic Acids on Gut Microbiota and Blood Profile of Broilers
Introduction
Intensive poultry production has been adjudged the best system of production to achieving better productivity [1]. Higher productivity is attained because more of the energy generated from digestion is channeled to production rather than maintenance [2]. However, still under the system, poultry farmers and nutritionists are faced with challenges to improve feed utilization for better performance. The gut environment (pH, viscosity and bacterial population) could affect feed utilization; hence nutritionists are adopting certain strategies to favor the gut mechanism to process the feed. Such strategy is addition of feed additives to diets and one of the earliest feed additives used is pharmaceutical antibiotics [3], but the practice is currently either restricted or banned in certain countries because of reported problem of antibiotic resistance [4].
Nevertheless, the concern about the antibiotic resistance in the food chain has necessitated nutritionists to look for alternatives for pharmaceutical antibiotics such as probiotics, prebiotic yeast culture, essential oils and spices [5]. Organic acids have also been suggested as one of the feed additives to modulate the gut environment for proper feed utilization [6,7]. However, it is pertinent to note that the search for alternatives to antibiotics should not be at the detriment of the health of the animals. Such products should not be such that will impact negatively on the blood composition of the chickens. Blood perform vital role in the body which include functions such as supplying oxygen and nutrients to tissues, removing waste, transporting hormones and other signals throughout the body, and regulating body pH and core body temperature [8]. Hence any feed additive as alternative for antibiotics should not jeopardize these important functions. Therefore, the objective of this research was to determine the dietary effect of organic acids on digesta pH, viscosity, bacteria population and blood composition of broiler chickens under production conditions.
Materials and Methods
Site of experiment
The experiment was conducted at the Teaching and Research Farm of Department of Nutrition and Forage Science of the Michael Okpara University of Agriculture Umudike; Abia State, Nigeria (latitude 5° 281N and longitude 7° 321E) with average rainfall of 2000mm. The average relative humidity during the experiment was over 72% and average ambient temperature of 28 °C.
Experimental design
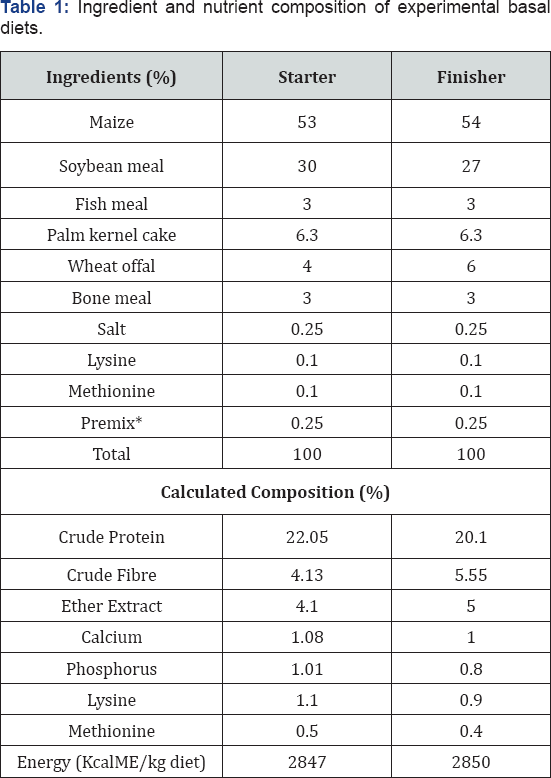
*Starter premix supplied/kg diet: Thiamine 2mg; Riboflavin 6mg; pyridoxine 4mg; Niacin 40mg; cobalamine 0.05g; Biotin 0.08mg; choline chloride 0.05g; Manganese 0.096g; Zinc 0.06g; Iron 0.024g; Copper 0.006g; Iodine 0.014g; Selenium 0.24mg; Cobalt 0.024mg and Antioxidant 0.125g.
Finisher premix supplied per kg diet: Vitamin A 10; 0001.u.; vitamin D3 12; 0001.u. Vitamin E 201.U.; Vitamin K 2.5mg; thiamine 2.0mg; Riboflavin 3.0mg; pyridoxine 4.0mg; Niacin 20mg; cobalamine 0.05mg; pantothenic acid 5.0mg; Folic acid 0.5mg; Biotin 0.08mg; choline chloride 0.2mg; Manganese 0.006g; Zinc 0.03g; Copper 0.006g; Iodine 0.0014g; Selenium 0.24g; cobalt 0.25g and antioxidant 0.125g. **Calculated
One hundred and fifty (150) day old chicks of Abor-Acre strain were used. There were divided into five treatments (T) replicated three times with 10 birds per replicate in completely randomized design (CRD). Each treated group received acetic acid, butyric acid, citric acid or formic acid at 0.25% level of their diets, while the control group received neither of the organic acids. Organic acids were introduced during the finisher phase. Single starter diet without organic acids was formulated and fed to all the birds at the starter phase (Table 1). At the end of the fourth week, the birds were allotted to the treatment groups making sure they have similar average live weight according to [9]. At the finisher phase, a basal diet without organic acids (Table 1) which formed the control was formulated to represent treatment one (T1). To a kilogram of the basal finisher diet, 0. 25% of acetic acid, butyric acid, citric acid and formic acid were added to form T2, T3, T4 and T5 respectively. Feeding of experimental diets started at the fifth week. The birds were vaccinated against Newcastle and Gumboro diseases as directed by a veterinary officer.
Determination of digesta pH, viscosity and bacteria load
A pH meter (PHep, Hanna Instruments, Italy) was used to determine the pH by collecting 1g of digeta from different segments of the gut according to Lee [10]. For viscosity, 5g of digesta obtained from the duodenum, ileum and caecum was used according to [11]. A Viscometer (Bohlin CS 50 Rheometer, manufactured by Bohlin Reologi, Muhlacker, Germany) was used. For bacteria load, 1.0g of digesta from the different segments was incubated at 37 °C for 24 hours and thereafter subjected to serial dilution technique according to [12]. Blood was collected and both haematological and serum chemistry analyses were carried out according to [12]. Blood collection was through the jugular vein by the use of hypodermic syringe into a 10ml capacity clinical Mackartney bottles containing dipotassium salt of ethelene-diamine-tetra-acetic acid (EDTA) as anticoagulant. Containers containing blood samples for serum biochemistry had no EDTA.
Data transformation and statistical analysis
Bacteria count expressed in colony forming units (CFU) were transformed using Log10 according to Alshawabkeh [6]. All Data collected were subjected to analysis of variance (ANOVA). Significant means were separated using Duncan New Multiple Range Test according to Hernandez [13].
Results and Discussion
Haematological indices
Results of haematological parameters (Table 2) indicate that feeding of acid treated diets did not significantly (P>0.05) alter parameters such as red blood cells, packed cell volume, mean corpuscular haemoglobin concentration, haemoglobin and white blood cells which agreed with [14]. In any case, significant (P<0.05) difference was recorded in mean corpuscular volume. The value of mean corpuscular volume was significantly (P<0.05) reduced by formic acid compared to others and control. This is in disagreement with Banerjee [15] who reported in the contrary regarding mean corpuscular volume and red blood cells. All the parameters fell within the normal range according to Paul [16] who reported the range of white blood cells to be 9-31 x 103/ mm3, red blood cells 2-4 x 106/mm3, haemoglobin 7-13g/100ml, packed cell volume 2 5-45%, mean corpuscular haemoglobin 3357%, mean corpuscular haemoglobin concentration 26-35% and mean corpuscular volume 90-140mg/100ml.

abc means along the same column with different superscripts are significantly different (P<0.05).
MCH: Mean Corpuscular Haemoglobin; MCHC: Mean Corpuscular Haemoglobin Concentration; MCV: Mean Corpuscular Volume; SEM: Standard Error of Means; CON: Control; AA: Acetic Acid; BA: Butyric Acid; CA: Citric Acid; FA: Formic Acid; WBC: White Blood Cells; RBC: Red Blood Cells; PCV: Packed Cell Volume; Hb: Haemoglobin
Blood Chemistry
The result of feeding acid treated diets as shown in Table 3, there were no significant differences (P>0.05) in protein, urea, cholesterol, creatinine, glucose, alkaline phosphatase and alanine amino transferase. Nevertheless, significant difference (P<0.05) was recorded in aspartate amino transferase (AST). The level of AST was higher in acetic and butyric acid groups (P<0.05) compared to the control which in turn posted the same level with citric and formic acid groups. The results were in consonant with that reported by Banerjee [15] except in cholesterol and aspartate amino transferase. Reports of Banerjee [15] showed a significant reduction in cholesterol level using all the tested organic acids. However, it could be because they fed the acids starting from the starter phase, thus the longer period of exposure to these acids could have resulted in the reduction. All the indices did not deviate from normal range Paul [16] reported in mg/100ml total protein 5-7, cholesterol 52-148, glucose 125-200, albumin 2-3.5, urea 0.5-6 and in (iu/l) alkaline phosphatase 25-44, alanine amino transferase 10-37, aspartate amino tranferase 88-208. This means that feeding of organic acid treated diets did not disrupt the biochemical processes of the broilers which would have caused these values to deviate from the normal range.
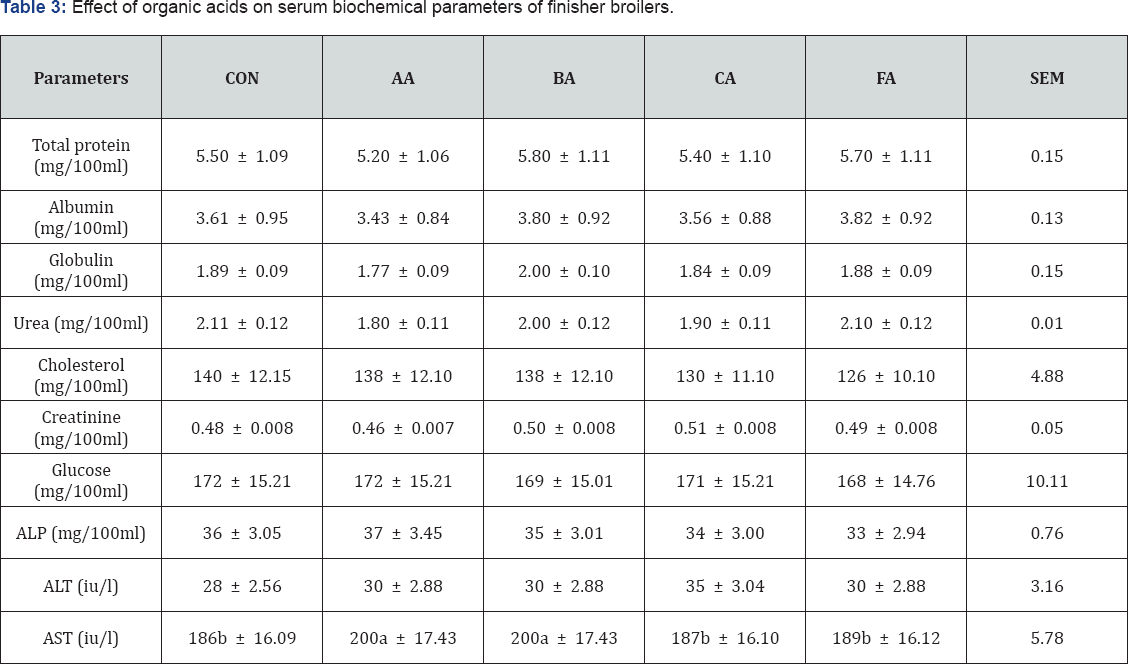
abc means along the same column with different superscripts are significantly different (P<l0.05).
SEM: Standard error of means; CON: Control; AA: Acetic Acid; BA: Butyric Acid; CA: Citric Acid; Formic Acid; ALP: Alkaline Phosphatase; ALT Alkaline Amino Transferase; AST: Aspartate Amino Transferase
Digesta pH
Table 4 shows that dietary exposure of broiler chickens to diets treated with organic acids significantly (P<0.05) influenced the pH at different segments of the gastro intestinal tract examined except the crop. Dietary feeding of organic acid treated diets caused pH in the proventriculus of acetic acid group of chickens to significantly (P<0.05) reduce more than the control. In the gizzard, it was noticed that the digesta of all the acid treated dietary groups was more acidic than the control (P<0.05). No significant differences existed within the acid treated groups. However, down to the duodenum, there were no significant differences (P>0.05) between the pH of the control group and those of the organic acid treated groups except the butyric acid treated group whose digesta was significantly (P<0.05) more acidic than the control. In the same segment, there were no significant (P>0.05) differences among the acid treated groups. At the ileum acid treated diets did not significantly (P>0.05) reduce digesta pH. In the caecum all the acids reduced the pH significantly. Use of organic acid treated diets to reduce digesta pH has been stressed [17], thereby supporting this work.
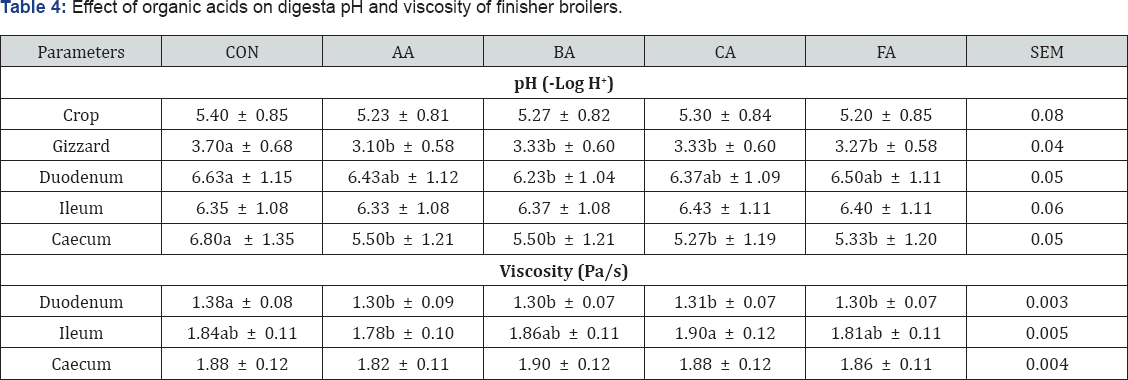
abc means along the same column with different superscripts are significantly different (P<0.05).
SEM: Standard Error of Means; CON: Control; AA: Acetic Acid; BA: Butyric Acid; CA: Citric Acid; FA: Formic Acid
Digesta Viscosity
Table 5 also shows the viscosity at different segment of the gastro intestinal tract. The organic acid treated diets significantly (P<0.05) reduced viscosity at the duodenum. At the ileum and caecum, there were no significant differences between the acids and the control. This agreed with Dibner [4] who reported that organic acids could be used to reduce digesta viscosity.

abc means along the same column with different superscripts are significantly different (P<0.05).
SEM: Standard error of means; CON: control; AA: Acetic Acid; BA: Butyric Acid; CA: Citric Acid; FA: Formic Acid
Bacterial Load of Gastro Intestinal Tract (GIT)
The effects of acid treated diets on bacteria population at both the foregut and hindgut of the GIT (Table 5 & 6) show the diets in comparison with the control significantly (P<0.05) reduced total bacteria count in all the segments except in the large intestine. In similar vein, Salmonella population was significantly (P<0.05) reduced in all the segments except in the ileum and large intestine. The ability of the organic acids to reduce Salmonella in the GIT was reported by Alshawabkeh [6] and Van Immerseel [18]. Report of Canibe [19] indicated that medium chain organic acids could be used to reduce salmonella in the gastro intestinal tract of poultry .
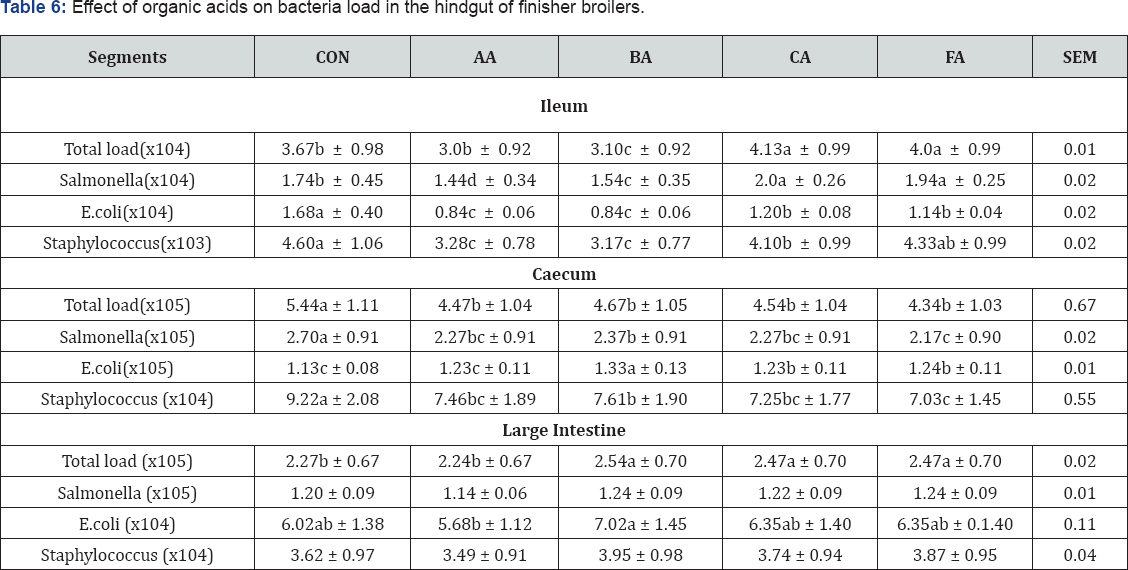
abc means along the same column with different superscripts are significantly different (P<0.05).
SEM: Standard Error of Means; CON: Control; AA: Acetic Acid; BA: Butyric Acid; CA: Citric Acid; FA: Formic Acid
Further observations indicated that E. coli population was also significantly (P<0.05) reduced by organic acids in all the segments, but not in the large intestine. Similar results were noticed in the number of Staphylococcus which was significantly reduced by the organic acids in all the segments. However, the number was not significantly influenced by the acids in the large intestine in comparison with the control. This work is in agreement with the report of Sun [20] and Yuossef [21] that organic acids were good antibacterial agents in the GIT. In their own report Russell [22] specifically noted that E. coli and coli forms population were significantly (P<0.05) reduced in the gastro intestinal tract of broiler chickens and concluded that acidifiers can be used as potential alternatives to antibiotics in broiler diets. The poor antibacterial activity of the organic acids in the large intestine could be that as the diets moves down the GIT they were diluted thus reducing their potency. This result could be linked to the lower pH levels observed in the GIT of the chickens (Table 4). But this cannot be said of the crop where the control significantly recorded the highest bacterial load despite the fact that there was no significant difference in pH. However, investigation has shown a strong bactericidal effect of organic acids without significantly decreasing the pH value in the gut [23]. Another good reason is that the addition of the organic acids in the diet could have reduced the bacterial load of the diet before being consumed by the birds. This result agreed with the report of Adil [24].
Conclusion
The different dietary organic acids tested indicated antibacterial actions at different segments of the gastro intestinal tract and did not impart negatively on the blood profile. They reduced digesta pH and digesta viscosity indicating that they could be used to improve on digestibility and nutrient utilization by broiler chickens. Therefore the organic acids could act as alternatives to pharmaceutical antibiotics and are recommended for inclusion in diets for broiler chickens.
Acknowledgement
This research was funded by the University of Uyo, Uyo, Nigeria and Tertiary Education Trust Fund (TETFund), Nigeria. The authors are grateful.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/open-access.php
For more articles in Open Access Journal of Dairy & Veterinary sciences please click on: https://juniperpublishers.com/jdvs/index.php



Comments
Post a Comment